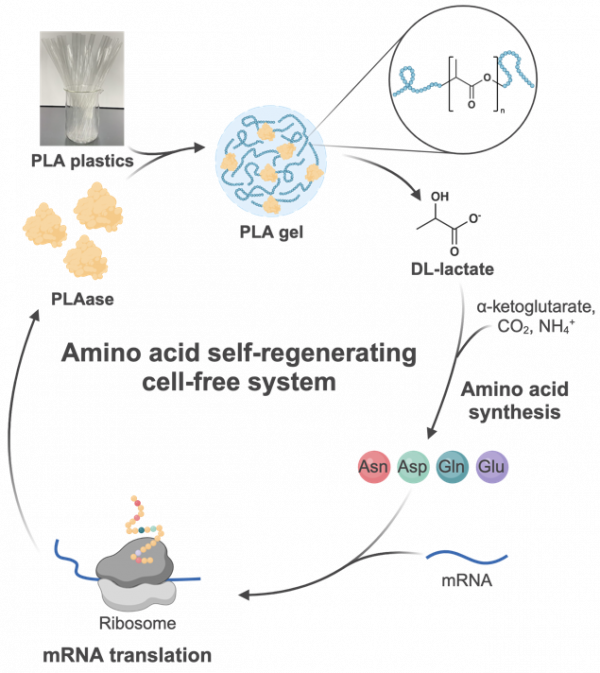
Researchers have developed a cell-free system that converts plastic waste into amino acids, the building blocks of proteins. This innovative system uses enzymes to break down polylactic acid (PLA) plastic, CO2, and ammonium, synthesizing amino acids that are then used to produce proteins without cells. This approach not only demonstrates the sustainable valorization of plastic waste but also creates a self-regenerating artificial cell that can synthesise its own materials from waste, emulating natural metabolic cycles.
The continuous growth of global plastic production, particularly polyesters like polylactate (PLA), has led to uncontrollable plastic pollution and subsequent negative environmental impacts.Although PLA is a biodegradable polyester derived from renewable biomass, its natural degradation can take decades. With the rapidly increasing global production of PLA and the limitations of composting and incineration methods, there is an urgent need to develop efficient recycling and upcycling technologies to address this issue.
In a recent study, an international team led by researchers from the Earth-Life Science Institute (ELSI) and National Central University has developed an innovative artificial biochemical system that transforms PLA plastic wastes into protein building blocks for sustainable biosynthesis. This “plastic-eating” cell-free system couples an enzymatic cascade that synthesises amino acids from PLA, CO2, and ammonium, with a cell-free protein production platform.
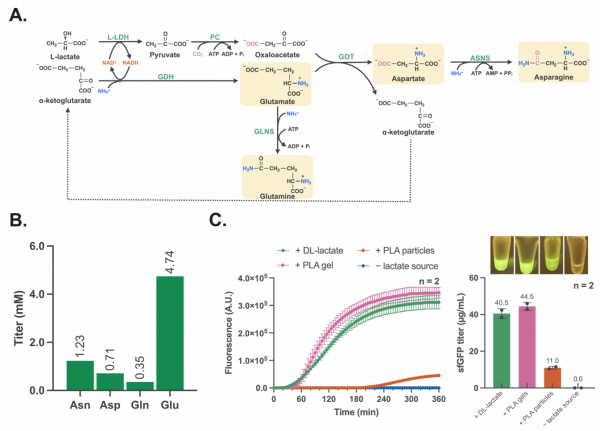
The researchers first established a one-pot multienzyme cascade that converts L-lactate (PLA monomer), α-ketoglutarate, CO2, and ammonium into four amino acids including asparagine, aspartate, glutamine, and glutamate via pyruvate as the intermediate (Figure 2A). This cascade enables the concurrent generation of an amino acid backbone building block (pyruvate) and a shared amine group donor (glutamate) for downstream amino acid synthesis. the researchers successfully produced these four amino acids simultaneously through the multienzyme cascade, with titers ranging from 0.35 mM for glutamine to 4.75 mM for glutamate (Figure 2B).
Next, they integrated this amino acid self-regenerating cascade with a cell-free protein synthesis system lacking those four amino acids. By adding PLA hydrolase (PLAase) along with a messenger RNA (mRNA), the system could produce proteins like green fluorescent protein by incorporating the plastic-derived amino acids. (Figure 2C).
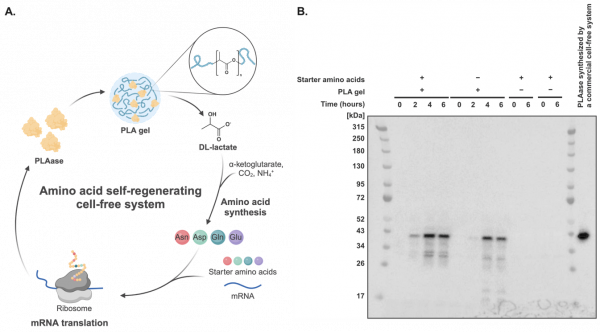
Finally, as a proof of concept for a “life-like” self-regenerating system, the researchers introduced a PLAase-coding mRNA to the metabolic cascade. This enabled the cell-free system to hydrolyze PLA to lactate for amino acid synthesis, which could then be incorporated into newly synthesized PLAase, forming a self-regenerating cycle in vitro. (Figure 3).
This novel approach represents a significant milestone in the development of a partially self-regenerating cell-free system that couples PLA catabolism with mRNA translation via amino acid synthesis. It demonstrates how plastic waste can be upcycled into higher-value biomolecules in an environmentally sustainable manner, reducing reliance on petrochemical sources. Furthermore, it serves as a crucial step towards achieving a “self-regenerating” artificial cell that synthesises its own building blocks from waste feedstocks, mimicking life-like metabolic cycles coupled to the central dogma of molecular biology.
The researchers anticipate that this PLA-eating cell-free system will serve as an efficient platform for future studies on the synthesis and evolution of enzymes and polypeptides, as well as investigations into cell-wide metabolic networks, paving the way for innovative solutions to address the global plastic pollution crisis.
| Title of the paper | ACS Catalysis |
| Title of the paper | Amino Acid Self-Regenerating Cell-Free Protein Synthesis System that Feeds on PLA Plastics, CO2, Ammonium, and α-Ketoglutarate |
| Authors | Shota Nishikawa‡[a,b], Wen-Chi Yu‡[c], Tony Z. Jia[a,d], Ming-Jing He[c], Anna Khusnutdinova[e], Alexander F. Yakunin[e], Yin-Ru Chiang[f,g], Kosuke Fujishima[a,h]*, and Po-Hsiang Wang[c,i]* |
| Affiliations | [a]Earth-Life Science Institute, Tokyo Institute of Technology, Tokyo 152-8550, Japan [b]School of Life Science and Technology, Tokyo Institute of Technology, Tokyo 152-8550, Japan [c]Graduate Institute of Environmental Engineering, National Central University, Taoyuan 320, Taiwan [d]Blue Marble Space Institute of Science, Seattle, Washington 98104, United States [e]Centre for Environmental Biotechnology, School of Natural Sciences, Bangor University, Bangor, Gwynedd, LL57 2UW, UK [f]Biodiversity Research Center, Academia Sinica, Taipei 115, Taiwan [g]Department of Agricultural Chemistry, National Taiwan University, Taipei 10617, Taiwan [h]Graduate School of Media and Governance, Keio University, Fujisawa 252-0882, Japan [i]Department of Chemical and Materials Engineering, National Central University, Taoyuan 320, Taiwan |
| DOI | 10.1021/acscatal.4c00992 |
| Online published date | 2 May 2024 |
