Sugars are important building block in the life and can undergo a plethora of intra- and inter-molecular reactions in aqueous media. Among the important intramolecular processes are carbonyl migration and accompanying epimerization. In this paper, the team of researchers, including scientists of ELSI, discovered that the carbonyl migration-epimerisation process can take place along the entire carbon chain of D-tetroses under potential prebiotic conditions. This study will help understand the stability and chirality of proto-sugars after forming.

Sugars perform several important roles in life, such as energy production and storage, structural components of membranes and cell walls, and integral parts of RNA/DNA. Sugars are complex chiral molecules and can undergo a plethora of intra- and inter-molecular reactions. Among the important intramolecular processes are carbonyl migration and accompanying epimerisation. In biochemistry, carbonyl migration is also an important mechanism for sugar metabolism. For example, enzyme-catalysed carbonyl migration occurs in core metabolic pathways, such as isomerisation of fructose 6-phosphate to glucose 6-phosphate in gluconeogenesis, and ribulose 5-phosphate to ribose 5-phosphate in the pentose phosphate pathway. It is possible that this fundamental biochemical transformation may trace its origins back to an era that preceded enzymatic catalysis. However, carbonyl migration in prebiotic sugar chemistry is relatively unexplored.
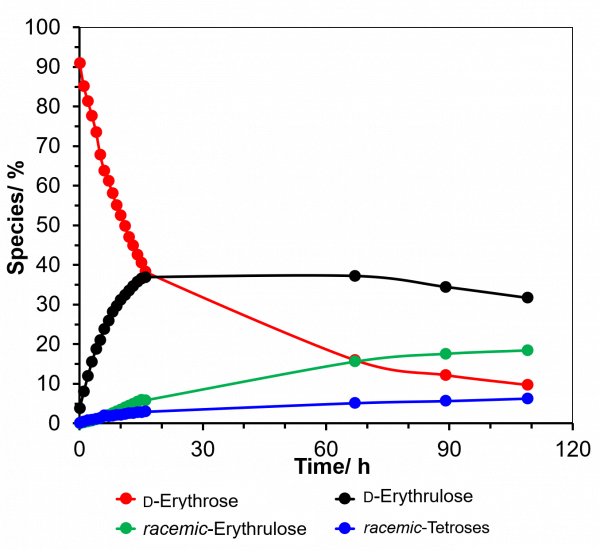
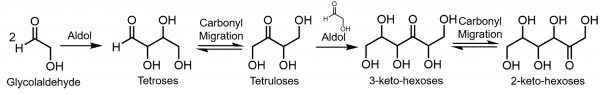
Here, the authors employed d-erythrose (a chiral pure tetrose) to study carbonyl migration. After d-erythrose was dissolved in bicarbonate buffer under mild basic conditions, a mixture of isomers, including d-erythrulose, racemic-erythrulose and racemic-tetroses was formed over time. These isomers were the result of carbonyl migration from C1 to C2, C3 and C4 in the erythrose carbon chain, respectively (Figure 1). This observation show that carbonyl migration can occur down the entire carbon chain of d-tetroses. Over a period of 109 hours, the carbonyl migrations lead to a mixture of 10% d-erythrose together with 32% d-erythrulose, 18% racemic-erythrulose, and 4% racemic-tetroses (Figure 2).
This study will hopefully help future prebiotic chemists understand the effect of carbonyl migrations in prebiotic sugar chemistry. “Our results reveal a further challenge with respect to the origin of sugar-chirality, since carbonyl migration effectively scrambles the configuration at each asymmetric center. Even if pure sugar could be synthesised prebiotically, its concentration and purity would continually decrease overtime as a consequence of carbonyl migration, ultimately scrambling its d-handedness.”, says co-corresponding author Ruiqin Yi, who is a research scientist at ELSI.
In addition to compromising the chirality of sugars, the authors also found that carbonyl migration has a significant effect on the nature of the product distribution under plausible prebiotic scenarios (such as formose reaction). The authors tested the aldol-reaction of glycolaldehyde and observed that 2-keto-hexoses were the major C6 sugars together with erythrulose. This result indicates that most of the newly formed tetroses were converted to tetruloses via carbonyl migration, followed by aldol reaction with glycolaldehyde to yield 3-keto hexoses and further carbonyl migration of 3-keto hexoses to afford 2-keto-hexoses (Figure 3).
| Journal | Chemistry—A European Journal |
| Title of the paper | Erythrose and Threose: Carbonyl Migrations, Epimerizations, Aldol, and Oxidative Fragmentation Reactions Under Plausible Prebiotic Conditions |
| Authors | Ruiqin Yi1,3*, Ryan Kern1, Pamela Pollet1, Huacan Lin2, Ram Krishnamurthy2*, Charles L. Liotta1* |
| Affiliations | 1.School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332, Unites States 2. Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037, Unites States 3. Earth-Life Science Institute, Tokyo Institute of Technology, 2-12-1-IE-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan |
| DOI | 10.1002/chem.202202816 |
| Online published date | 11 November 2022 |
