The RNA world hypothesis holds that RNA dominated the chemistry of the first primitive cells. However, the claim that RNA was the first polymer to arise on early Earth is more controversial. Alternatively, xeno nucleic acids, such as glycol nucleic acid, are also candidates for the original genetic polymers. This study demonstrates how glycol nucleic acid monomers can be combined with simple dicarboxylic acid linkers to generate linear and branched alternating co-polymers via evaporative drying. Linear polymers may be more likely to perform information-carrying-related functions, while branched polymers could be more catalytic.
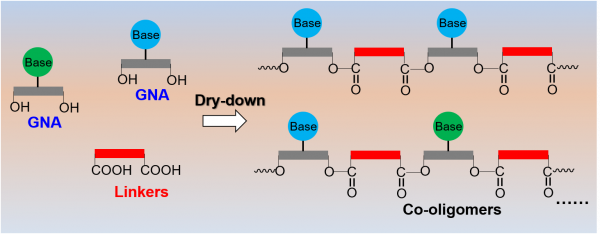
It is generally believed that the chemical origins of life on Earth required the abiotic synthesis of “genetic polymers” for information storage. Chemists have long sought to understand how such genetic polymers could have been generated in the primitive environment. One popular model, the RNA world hypothesis, suggests RNA could have been the original genetic polymer. However, the formation of RNA in early planetary environments is fraught with difficulties. One big challenge for RNA polymerization is the formation of phosphodiester bonds, which is a thermodynamically unfavourable reaction in water. As alternative candidates to RNA, scientists have considered various xeno nucleic acids (XNAs), which contain alternative backbone structures. Some examples include threose nucleic acid (TNA), peptide nucleic acid (PNA), and glycol nucleic acid (GNA). Research led by Ruiqin Yi from the Earth-Life Science Institute (ELSI) at Tokyo Institute of Technology and H. James Cleaves II from Blue Marble Space Institute of Science has recently demonstrated the co-polymerization of GNA monomers with dicarboxylic acid linkers under plausible early Earth aqueous dry-down conditions. Researchers from the Earth-Life Science Institute, the German Aerospace Center and the University of New South Wales also assisted in the work.
To conduct this work, the team prepared aqueous solutions containing GNA monomers and various dicarboxylic acids and then dried them by heating (Figure 1). These conditions simulate evaporative environments, such as puddles and beaches, which were likely common on early Earth. As lead author Ruiqin Yi says, ‘Amino acids have been known to polymerize via wet-dry scenario, but this method is inefficient for RNA polymerization due to the thermodynamic unfavourable of phosphate ester formation. That is why we were looking for ways to make other potential primordial genetic polymers in wet-dry environments.’ The team members think that linkages, such as carboxylate esters, could be good candidates due to low free energy for their formation. As predicted, the team showed that co-oligomers containing up to eight GNA units long could be generated via just one simple cycle of dry-down when carboxylate esters were chosen as the linking bond type (Figure 1).
Interestingly, besides linear alternating oligomers, they also found the formation of branched oligomers when substituted dicarboxylic acids were used. The authors think the branched co-polymers can be considered a type of hybrid macromolecular structure, and the initial linker ratio and type might ultimately control their functions. This will hopefully help future studies explore the origins of hybrid polymer macromolecules in biochemistry (Figure 2). This interconnectivity increases the complexity of the polymers but also may afford them more functions than the individual polymers alone.
| Journal | Chemical Communications |
| Title of the paper | Alternating co-synthesis of glycol nucleic acid (GNA) monomers with dicarboxylic acids via drying |
| Authors | Ruiqin Yi,1* Tony Z. Jia,1,2 Markus Meringer,3 Luke K. Marshall,4 Chen Chen,1 Shawn Erin McGlynn,1 Albert C. Fahrenbach,4 and H. James Cleaves, II1,2* |
| Affiliations | 1. Earth-Life Science Institute, Tokyo Institute of Technology, Tokyo, Japan 2. Blue Marble Space Institute of Science, Seattle, WA 98154 3. Earth Observation Center, German Aerospace Center, Oberpfaffenhofen–Wessling, Germany 4. School of Chemistry, Australian Centre for Astrobiology and the UNSW RNA Institute, University of New South Wales, Sydney, NSW 2052, Australia |
| DOI | 10.1039/D2CC06818D |
| Online published date | 29 April 2023 |
