Polyester microdroplet protocells generated from prebiotically plausible dehydration/rehydration of 𝛼-hydroxy acid monomers exhibit major structural changes with even minor differences in salt-uptake from aqueous environments, shows a recent study led by ELSI scientists. The novel analytical strategy to measure salt–polyester interactions used in this study helps to overcome fundamental challenges faced by using purely conventional methods in origins of life research, paving the way for deeper insights into complex prebiotic chemistry and fostering further interdisciplinary discoveries in this realm.
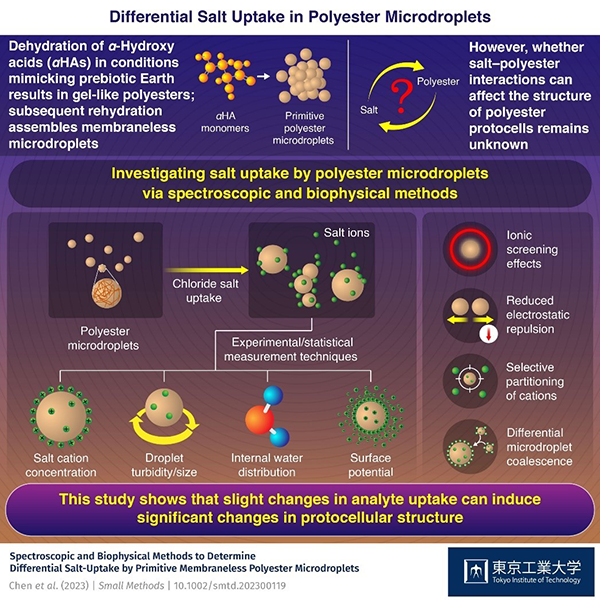
Figure 1. Overview of the study and the techniques used to measure each physical or chemical property. Credit: Tokyo Institute of Technology
In 2019, researchers from the Earth-Life Science Institute (ELSI) at Tokyo Institute of Technology, including Tony Z. Jia, Kuhan Chandru (now at National University of Malaysia), and H. James Cleaves II (now at Howard University) discovered that polyester microdroplets could be generated from dehydration and rehydration of simple mixtures of 𝛼-hydroxy acid (𝛼HA) monomers. These microdroplets were membraneless, and were proposed as protocell models which could have been primitive compartments that interacted with and took up various primitive analytes. One type of very abundant primitive analyte is salt, which is very common on modern Earth, and was also abundant on early Earth. Polyester microdroplets were observed to coalesce rapidly upon sodium chloride addition (perhaps as a way of primitive protocellular growth), but why this occurred remained a mystery. In particular, primitive molecules such as 𝛼HAs and polyesters, though not as commonly used by current living systems as amino acids, may have laid the ground for the evolution of primitive chemical systems that led to the origin of life on Earth (i.e., as “non-biomolecules”). Examining the interaction of polyesters with different prebiotic analytes such as salts and determining whether polyester droplets can uptake salts can provide insights into relevant functions exhibited by primitive compartments.
To figure out what could be causing the primitive coalescence process, a team of researchers led by Special Postdoctoral Researcher Chen Chen from RIKEN (formerly of ELSI, Tokyo Institute of Technology) and Specially Appointed Associate Professor Tony Z. Jia from ELSI at Tokyo Institute of Technology recently quantified salt uptake by polyester microdroplets, leading to more understanding about salt-driven primitive polyester microdroplet coalescence. Their breakthrough, published in Small Methods on May 18, 2023, repurposed existing spectroscopic and biophysical methods used in other fields to characterize salt uptake.
The microdroplets could indeed selectively partition salt cations, with potassium being more highly uptaken than others. In particular, the potassium/sodium ratio was more in-line with that which is observed in modern cells, rather than what would have been present in the early oceans (where sodium is much more abundant than potassium). Salt uptake also led to differential coalescence of microdroplets, likely due to reduced electrostatic repulsions between the microdroplets as a result of surface charge neutralization by the uptaken salts, which preferentially localized to the droplet surface. Even slight changes in salt-uptake could significantly affect protocell structure, which could potentially account for diversity in chemistries of primitive systems that emerged in different aqueous systems—ranging from freshwater to oceanic to hypersaline under-ocean brines.
“This study lays the basis for analyzing analyte uptake into polyester microdroplets, and may lead to further studies on other primitive analytes within this system, which will also lead to greater understanding of the relevance of polyester-based protocells at the origins of life,” remarks Chen.
The team also concluded that the adoption of a novel and highly sensitive strategy for analyzing salt uptake by polyester microdroplets widened the range of known primitive chemicals that could have had an effect on primitive protocell structure and function. This study in particular “borrowed” techniques from biophysics, chemistry, geochemistry, and even cosmochemistry. One of the major collaborators of this study came from the Institute of Planetary Materials at Okayama University, which utilizes a mass-spectrometric-based technique used in the study to analyze dust grains from extraterrestrial samples. If this technique can be used for both protocell research and planetary science, then surely other useful techniques that can straddle the line between adjacent fields exist as well!
“We hope that this study encourages origins of life researchers to repurpose and utilize different existing techniques in other fields to answer important questions in origins of life, an inherent interdisciplinary field,” says Jia.
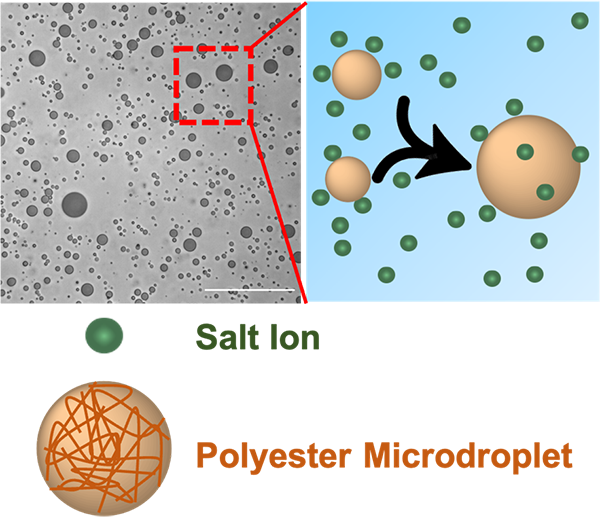
Figure 2. Structural representation of salt ion uptake by polyester microdroplets. Reprinted with permission from C Chen, et al. Spectroscopic and Biophysical Methods to Determine Differential Salt-Uptake by Primitive Membraneless Polyester Microdroplets. Small Methods, 2300119, 2023 under a Creative Commons License.
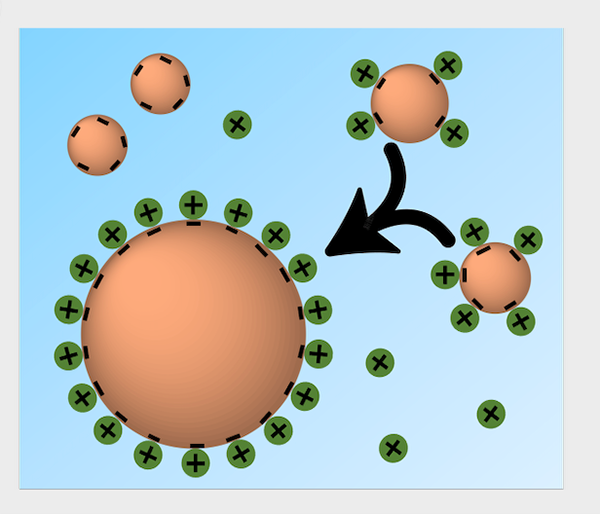
Figure 3.Salt cations (green) preferentially localize to the droplet (orange) surface. Reprinted with permission from C Chen, et al. Spectroscopic and Biophysical Methods to Determine Differential Salt-Uptake by Primitive Membraneless Polyester Microdroplets. Small Methods, 2300119, 2023 under a Creative Commons License.
| Journal | Small Methods |
| Title of the paper | Spectroscopic and Biophysical Methods to Determine Differential Salt-Uptake by Primitive Membraneless Polyester Microdroplets |
| Authors | Chen Chen1*, Ruiqin Yi1, Motoko Igisu2, Chie Sakaguchi3, Rehana Afrin1 , Christian Potiszil3, Tak Kunihiro3, Katsura Kobayashi3, Eizo Nakamura3, Yuichiro Ueno1,2,4, André Antunes5, , Anna Wang7,8,9,10, Kuhan Chandru11, Jihua Hao6,12, Tony Z Jia1,6* |
| Affiliations |
|
| DOI | 10.1002/smtd.202300119 |
| Online published date | 18 May 2023 |
