Lactic acid is not only a major byproduct of primitive synthetic processes such as spark discharge or UV irradiation, it also is a significant player in modern biology. Here, a research team led by ELSI researchers and collaborators shows that lactic acid, which is known to form polyesters and membraneless droplets upon a dehydration/rehydration cycle, may be more versatile and relevant to the origin of membraneless protocells than previously thought.
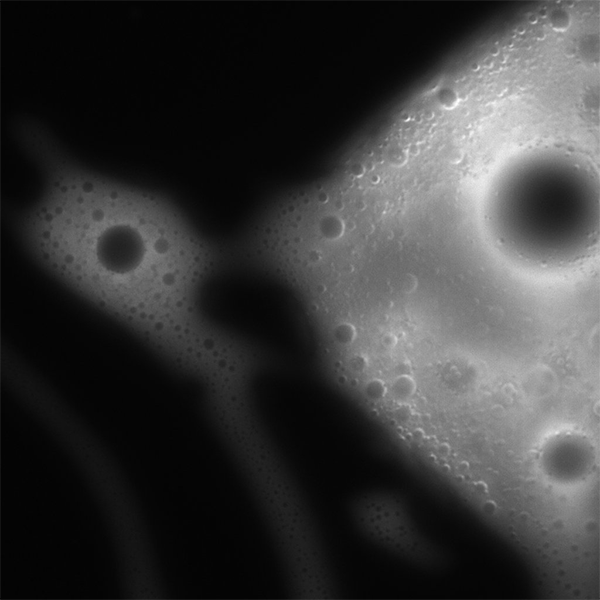
Lactic acid (LA) is an alpha hydroxyacid (AHA) that likely existed abundantly on early Earth, possibly as a result of a number of prebiotically plausible chemical or geological processes, such as atmospheric discharge, UV irradiation, or even meteoritic delivery. Its likely abundance on early and its major role in modern biological processes such as metabolism, has led researchers to postulate whether LA could have itself participated in chemistries on early Earth that eventually led to the origin of life. Indeed, previously, researchers at the Earth-Life Science Institute (ELSI) at Institute of Science Tokyo discovered in 2018 that LA and other AHAs can polymerise quite efficiently into polyesters through simple dehydration processes as a result of heat, something would have been possible on early Earth. These polyesters could then go on to form membraneless microdroplets upon rehydration (i.e., by precipitation). Thus, research regarding the structure and function of polyester microdroplets as potential protocells in the context of the origins of life has expanded since their first discovery in 2019. For example, ELSI researchers have discovered that polyester microdroplets can not only segregate biopolymers such as nucleic acids, they can even selectively uptake various salts.
However, while research on polyester microdroplet functions is exciting and impactful, all such research sits on a foundation of basic characterisation of the AHA/polyester system. Similarly, much of the functional research to date has focused on a specific AHA—phenyllactic acid (PA)—due to its ability to polymerize and subsequently assemble into microdroplets; for example, previous research has shown that PA can polymerise and form microdroplets at fairly low reaction concentrations and volumes. To that end, the authors of the study decided that more detailed characterization of the range of the conditions by which LA, which is likely to have been more abundant than PA on early Earth, can polymerise and form membraneless microdroplets on early Earth.
Thus, the authors first sought to characterise LA polymerisation and subsequent microdroplet formation in the presence of increasing concentrations of various salts, and found that although intermediate-to-high concentrations of divalent salts, such as calcium or magnesium chloride, appeared to inhibit LA polymerisation, monovalent salts had no such effect. This suggests that perhaps even primitive environments significantly high in monovalent salts would not inhibit LA polymerisation and subsequent membraneless microdroplet assembly.
Next, the authors proceeded to probe LA polymerisation at low reactant concentrations and reaction volumes. While LA exhibited the ability to polymerise and subsequently assemble into microdroplets at quite low reaction volumes, polymerisation appeared to be inhibited at low LA reactant concentrations down to the millimolar range. This is in contrast with PA, which appeared to still exhibit robust polymerisation even at low reactant concentrations in the same range. The authors postulate that this difference could stem from differences in evaporation rates between LA and PA. The boiling point of LA (~120 °C) is quite low compared to that of PA (~360 °C); as the dehydration synthesis reactions are performed at 80 °C, around half of the initially present LA is volatilised throughout the evaporative process, while nearly all of the PA remains present in the reaction vessel. Study co-corresponding author Specially Appointed Associate Professor Tony Z. Jia of ELSI exclaims, “Nevertheless, the study still revealed that LA is much more versatile than originally thought, and can still polymerize and assemble into membraneless droplets even in more extreme conditions, such as those on early Earth where material availability could be limited.”
“This is an important study in a series of systematic characterisation studies of the AHA/polyester system, which is essential to demonstrate the robustness and plausibility of the system/chemistries on early Earth,” explains research scientist Mahendran Sithamparam of the Chandru Lab, who was a PhD student of National University of Malaysia (UKM) at the time of the research and is a co-first author and performed some of the research during three research visits to the Jia Lab at ELSI funded by the ELSI Director’s Office Visitor (Brain Exchange) Programme from 2023 to 2025 (the same project also funded a visit by co-first author former master’s student Ming-Jing He of National Central University to the Jia Lab at ELSI in 2023). Study co-first-author master’s student Navaniswaran Tharumen of the Chandru Lab of UKM also says, “this study is also relevant to guide my future work on polyesters, and will hopefully be of help not only to those in the labs currently working on this system, such as those which were a part of this study, but to others around the world interested in starting primitive polyester research as well!”
Additional notes: The ELSI Director’s Office Visitor (Brain Exchange) Program no longer funds visits to ELSI for students or those without a PhD. Jia would like to acknowledge all of the co-authors of the paper for pushing the project forward while he was on long-term paternity leave from 2023–2024.

| Journal | Polymer Journal |
| Title of the paper | A deeper dive into primitive polylactate polymerization and microdroplet assembly under restrictive early Earth conditions |
| Authors | Mahendran Sithamparam1,†, Ming-Jing He2,†, Navaniswaran Tharumen1,†, Rehana Afrin3, Niannian Ding3,4, Chen Chen5, Ruiqin Yi6, Po-Hsiang Wang2,7,*, Tony Z. Jia3,8,*, Kuhan Chandru1,9,10* |
| Affiliations | 1Space Science Center (ANGKASA), Institute of Climate Change, National University of Malaysia, Bangi, Selangor, 43650, Malaysia 2Graduate Institute of Environmental Engineering, National Central University, No. 300, Zhongda Road, Zhongli District, Taoyuan City 320, Taiwan 3Earth-Life Science Institute, Institute of Science Tokyo, 2-12-1-IE-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan 4China University of Geosciences, Xueyuan Road 29, Haidian District, Beijing, 100190, China 5Biofunctional Catalyst Research Team, RIKEN Center for Sustainable Resource Science (CSRS), 2-1 Hirosawa, Wako, Saitama 351-0198, Japan 6State Key Laboratory of Deep Earth Processes and Resources, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, 510640, China 7Department of Chemical Engineering and Materials Engineering, National Central University, No. 300, Zhongda Rd., Zhongli District, Taoyuan, 32001, Taiwan (R.O.C.) 8Blue Marble Space Institute of Science, 600 1st Ave, Floor 1, Seattle, WA 98104, USA 9Polymer Research Center (PORCE), Faculty of Science and Technology, National University of Malaysia, Bangi, Selangor, 43600, Malaysia 10Institute of Physical Chemistry, CENIDE, University of Duisburg-Essen, 45141 Essen, Germany †These authors contributed equally. |
| DOI | https://doi.org/10.1038/s41428-025-01048-2 |
| Online published date | 30 April 2025 |
